PI

Chih-Yu Chao, Professor, Department of Physics & Institute of Applied Physics, National Taiwan University
200kV-FEG transmission electron microscope
Most biological mechanisms of living organisms are carried out through large molecular assemblies in the range of 10 to 100 nanometers. Cryo-electron microscopy(cryo-EM) provides architecture of these "molecular machines" and extends the capabilities of structural biology. We aim to understand the molecular structure of these macromolecules which is not only essential for the comprehension of their function and mechanism, but can also provide clues for the developing therapeutics related to health and disease.
We have worked on many proteins using cryo-EM and image processing technique. The GroEL is a kind of molecular chaperone which assists nascent proteins to reach their native fold and prevents protein aggregation. Structural analysis of this protein(Figure 1.) shed light on understanding the cure for Parkinson's disease. The earthworm hemoglobins(Figure 2.) are highly cooperative oxygen-carrying proteins. We found that the oxygenation causes a radial expansion of the complex(Figure 3.), which initiates from the interaction between the F-helix and heme group(Figure 4.).
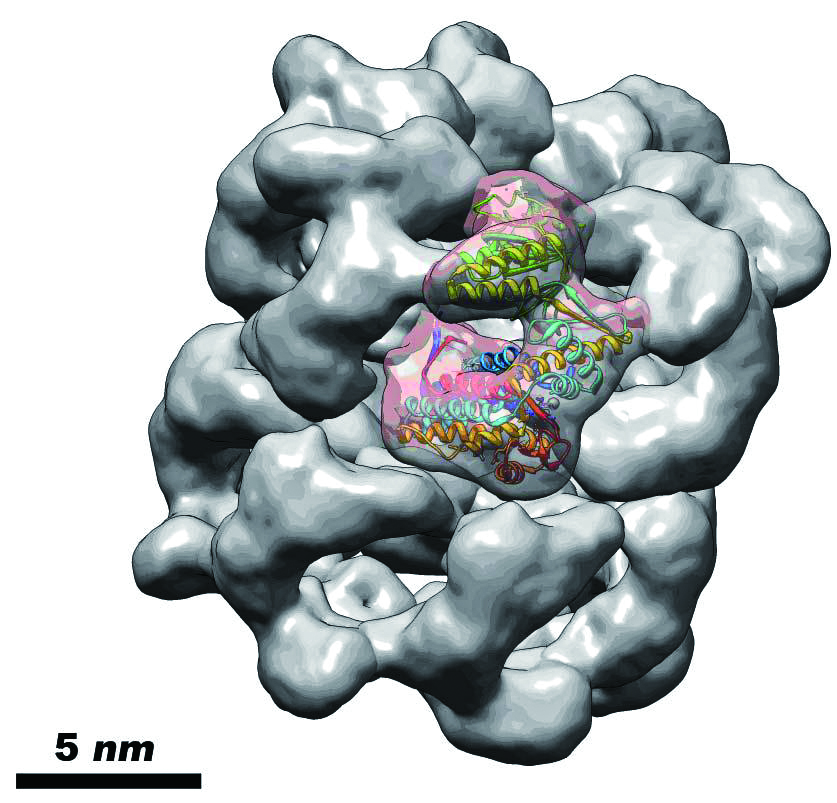
Figure 1. Cryo-EM structure of Escherichia coli GroEL

Figure 2. Cryo-EM micrograph of Lumbricus terrestris hemoglobin
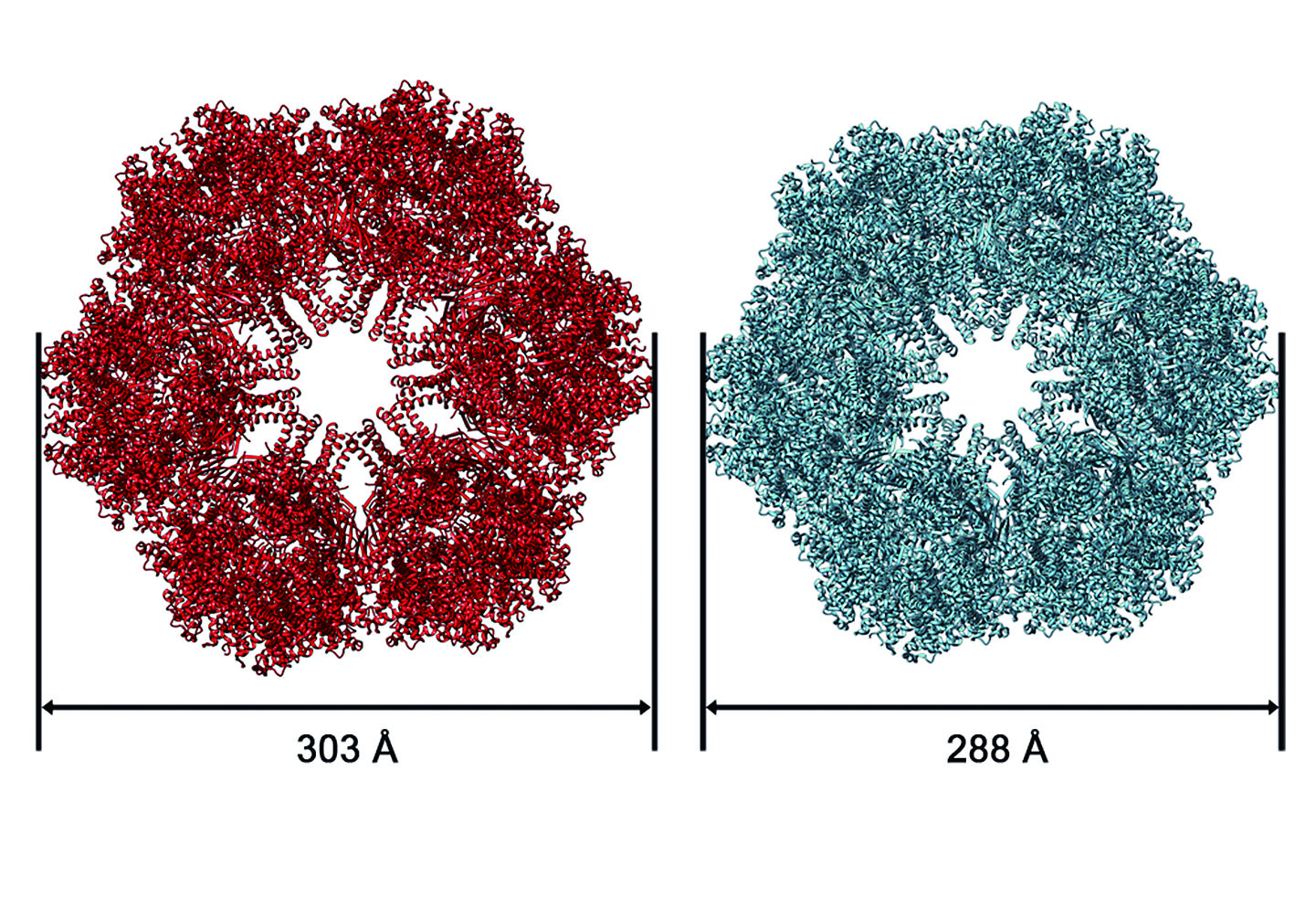
Figure 3. The CO and O2-bound states of the Lumbricus terrestris Hb are shown in cyan and red
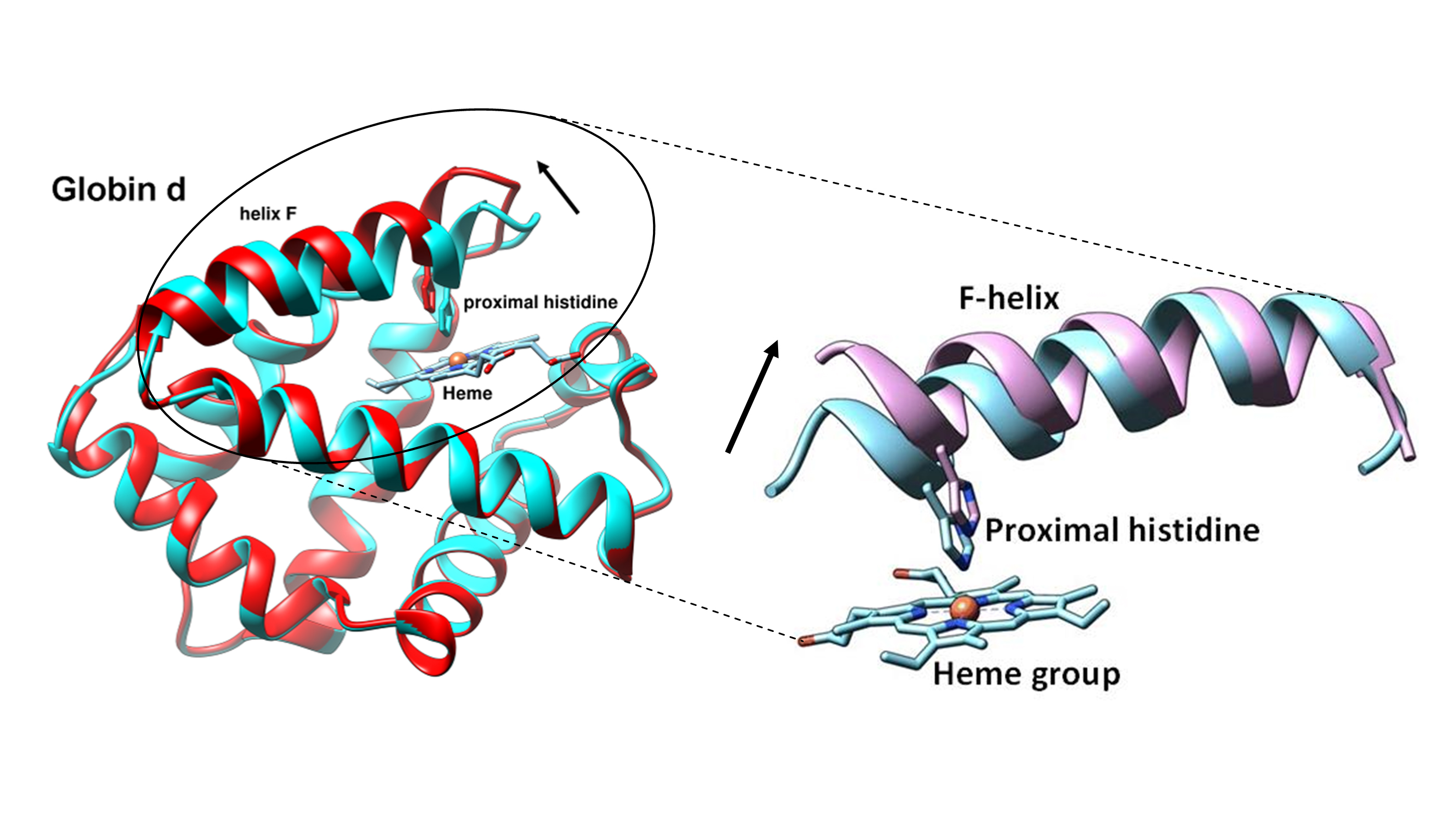
Figure 4. The helix F contains the proximal histidine tilts away from the heme group after binding O2 molecule

 繁體中文
繁體中文 English (UK)
English (UK)